How will my treatment be managed and monitored?
There are some tests you will need to have done regularly to monitor your condition. Some, such as scans, will check to see if your breast cancer is responding to treatment or progressing. Others will check for side effects of treatment. For example, there will be particularly regular monitoring for high blood sugar (hyperglycaemia), as this is a very common and serious side effect of this treatment.1
Getting these tests scheduled in can help you:
- Stick to your treatment plan
- Know what side effects to expect
- Find out how your cancer is responding to treatment.
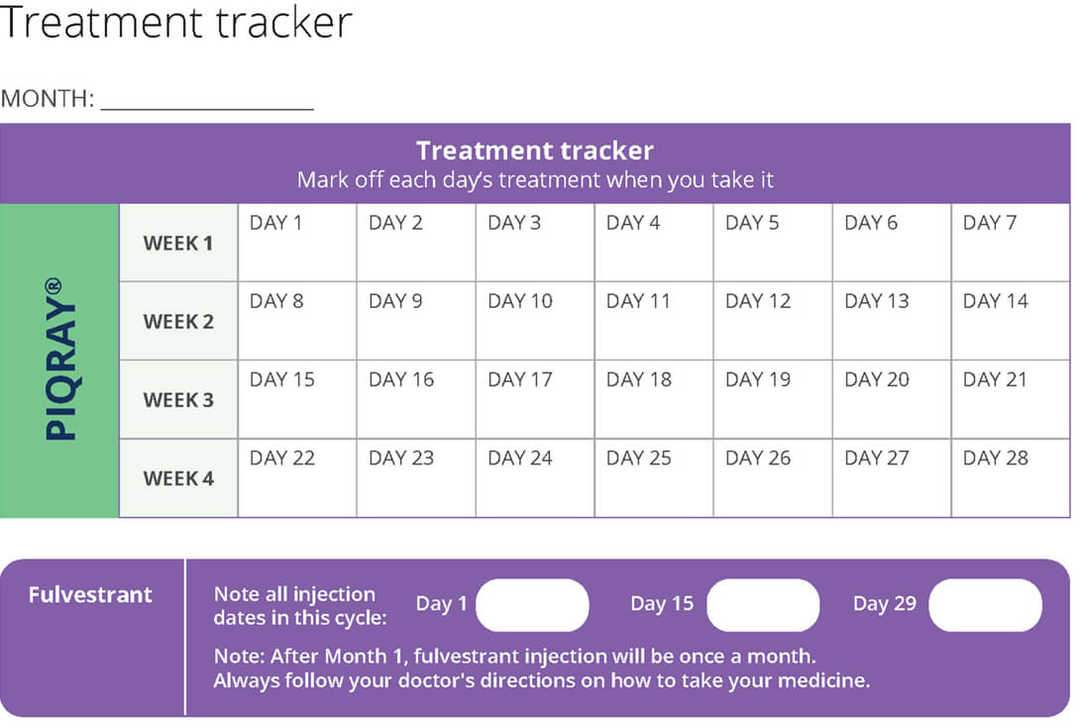
Treatment tracker
Using this treatment tracker will help you know where you are with your Piqray and fulvestrant doses.