PIQRAY®▼ (alpelisib)
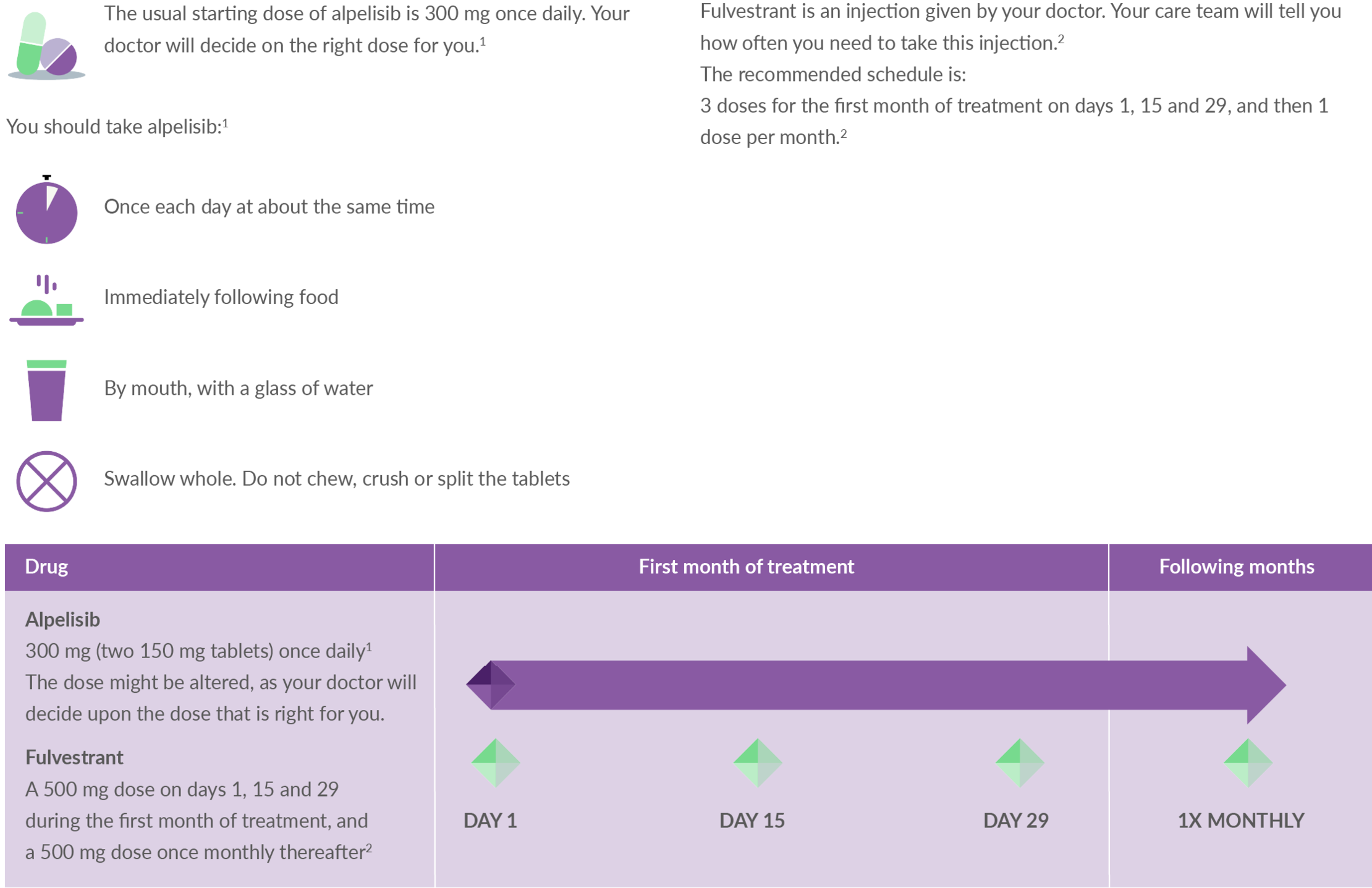
It is important that you take your medication exactly as it is prescribed. Find out more about the steps you need to take to ensure you are taking Piqray correctly
CTA
Learn more >

PIQRAY®▼ (alpelisib)
There are some circumstances that may affect your treatment with Piqray; it is important to know about these and the safety information associated with them so that your treatment is managed appropriately

PIQRAY®▼ (alpelisib)
There is a certain protocol to follow if you miss a dose of your Piqray. It is important to follow this to maximise your treatment

PIQRAY®▼ (alpelisib)
If you take too much Piqray by accident, you may experience some side effects. Find out more about what to expect and what to do next
CTA
Find out more >

PIQRAY®▼ (alpelisib)
Find out if there are any special storage instructions for Piqray